"Everything for CO2 retail applications" compendium

Despite already having been utilized in the early 1900s, carbon dioxide has only become widely used as a refrigerant in recent years. This is due to increasing interest in natural fluids, as well as legislation that, especially in Europe, aims to limit the use of synthetic refrigerants.
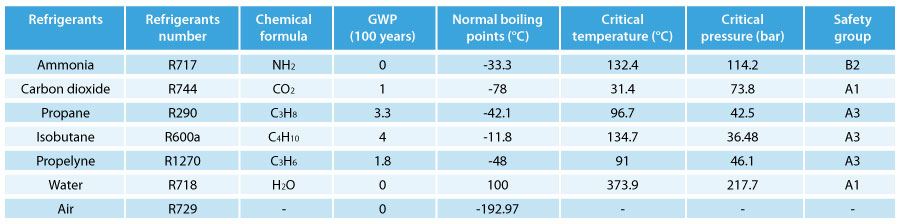
Widely available, even as a waste product from other processes, CO2 is much less expensive than traditional refrigerants. Together with this economic advantage are other benefits, such as low global warming potential (GWP =1, no impact on the ozone layer), no dangers relating to toxicity or flammability, and no need to recycle the gas at the end of system life.
CO2 has several thermodynamic properties that in many different applications can represent advantages and allow it to compete head-to-head with traditional refrigerants.
|
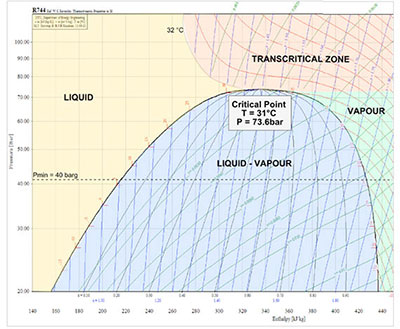
The main difference between carbon dioxide and synthetic fluids is that the critical point is 31.1 °C, a temperature that is easily reached in many different parts of the planet. At critical point, the density of the liquid and saturated gas are the same, while at higher temperatures there is no longer a boundary between the two phases, and this is referred to as supercritical state. Consequently, pressure and temperature are no longer related, meaning measures need to be adopted to keep these under control, optimize heat exchange and maximize efficiency. |
|
Types of cycles |
||
|
Literature gives the critical temperature for CO2 as around 31°C (87°F), while the critical pressure, again approximately, is 73 barg (1045 psig). CO2 systems operate in different ways according to whether they work above or below the critical point; in essence, in a subcritical system the temperature of the CO2 in the isothermal stage following compression of the fluid is below critical temperature, while in a transcritical system the temperature of the CO2 at the gas cooler outlet is above 31°C, and obviously evaporation temperature is lower.. |
||
|
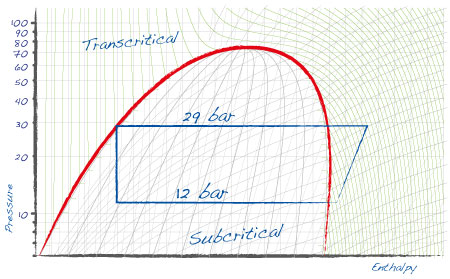
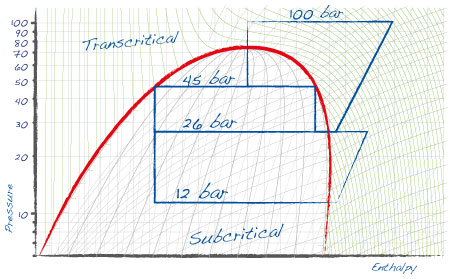
The simplest application of carbon dioxide as a refrigerant is in the subcritical cycle: CO2 is used in a secondary low temperature loop, either vapor compression (cascade system) or a pumped loop of liquid CO2. The primary cycle is managed using a traditional refrigerant, with the task of keeping condensing temperature for the CO2 cycle below the critical point, generally between -5 and -10 °C. |
A CO2 cycle that exchanges heat with the outside can also be adopted. This is referred to as the transcritical cycle, as in certain periods of the year outside temperature will be near or above the critical point of 31.1 °C. this involves the stage in which the compressed gas is cooled, which does not correspond to a constant "condensing" temperature. |
|